Medical Ultrasound Imaging
Monday, 13 May 2024
'Cine' p2 Searchterm 'Cine' found in 44 articles 3 terms [ • ] - 41 definitions [• ] Result Pages : • 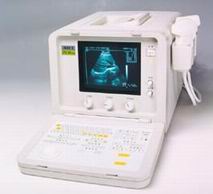 From SIUI Inc.;
From SIUI Inc.;'The CTS-385 Plus is designed for the diagnosis of liver, gallbladder, kidney, pancreas, thyroid, breast, uterus, bladder, ovary, etc. The system is a portable linear and convex unit for general application.' Features: 'High quality image Cineloop − 32-frame non-volatile storage capacity Probe frequency conversation option Computer image communication Various measuring function Foldaway keyboard for easy operation Dual probe connector'
Device Information and Specification
APPLICATIONS
See description above
CONFIGURATION
Portable, gray scale(256)
Linear and convex
PROBES STANDARD
1 * 2.5MHz ~ 5.0MHz trifrequency convex probe
2.5MHz to 10.0MHz, linear and convex, broad band, trifrequency
IMAGING OPTIONS
OPTIONAL PACKAGE
DATA PROCESSING
Pre-processing, correlation-processing, interpolation
H*W*D m
0.26 * 0.3 * 0.41
WEIGHT
10 - 13 kg
POWER REQUIREMENT
AC 220V/110V, 50Hz/60Hz
POWER CONSUMPTION
0.1 KVA
•
Different stages of the drug development and approval process in the USA, lead from preclinical trials (testing in animals), first application in humans through limited and broad clinical tests, to postmarketing studies.
Years
Test Population
Purpose
Success Rate
Preclinical Testing
3.5
Laboratory and animal studies
Assess safety and biological activity
5,000 compounds evaluated
Phase II
2
100 to 300 patient volunteers
Evaluate effectiveness, look for side effects
Phase III
3
1000 to 3000 patient volunteers
Verify effectiveness, monitor adverse reactions from long-term use
12 Total
By Dale E. Wierenga, Ph.D. and C. Robert Eaton Office of Research and Development Pharmaceutical Manufacturers Association 'In reviewing this report, it is important to keep in mind the realities of the drug discovery and development process. The U.S. system of new drug approvals is perhaps the most rigorous in the world. On average, it costs a company $359 million to get one new medicine from the laboratory to the pharmacist's shelf, according to a February 1993 report by the Congressional Office of Technology Assessment.' See also Phase 1 2 3 4 Drug Trials, Food and Drug Administration, and European Medicines Agency. • 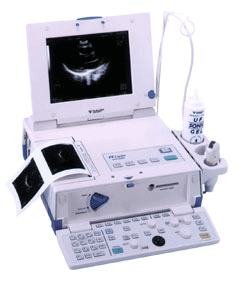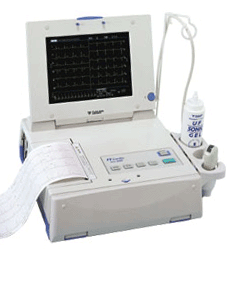 From Fukuda Denshi Co., Ltd.;
From Fukuda Denshi Co., Ltd.;'Ultrasound Scanner with built-in Electrocardiograph Realizes Diagnostic Ultrasound Imaging and 12-lead ECG examination with one unit Large 10.4-inch TFT color LCD adopted Cine memory of maximum 64 frames is available Four types of Color Scale Imaging facilitate identification of delicate signals High Frame Rate mode powerfully supports the Cardiovascular region Image data filing in IC card for data management and for easy comparison with past data M-mode continuous recording available with 3-ch ECG data from the cine memory A highly precise and quality printer is integrated Enhanced 12-lead ECG analysis report provided Hybrid and compact design ensures space saving' •
Regulations governing the output of diagnostic ultrasound have been largely set by the USA's Food and Drug Administration (FDA), although the International Electrotechnical Commission (IEC) is currently in the process of setting internationally agreed standards. The relevant national societies for ultrasound users (e.g. American Institute of Ultrasound in Medicine (AIUM), British Medical Ultrasound Society (BMUS)) usually have safety committees who offer advice on the safe use of ultrasound. In 1992, the AIUM, in conjunction with the National Electrical Manufacturers Association (NEMA) developed the Output Display Standard (ODS), including the thermal index and mechanical index which have been incorporated in the FDA's new regulations. Within Europe, the Federation of Societies of Ultrasound in Medicine and Biology (EFSUMB) also addresses safety and has produced safety guidelines (through the European Committee for Ultrasound Radiation Safety). The World Federation (WFUMB) held safety symposia in 1991 (on thermal issues) and 1996 (thermal and non-thermal issues), at which recommendations were proffered. The FDA ultrasound safety regulations from 1993 combine an overall limit of spatial peak time averaged intensity (I-SPTA) of 720 mW/cm2 for all equipment. A system of output displays allows users to employ effective and judicious levels of ultrasound appropriate to the examination. The output display is based on two indices, the mechanical index (MI) and the thermal index (TI). See also ALARA Principle, and Radiological Society of North America. •  [This entry is marked for removal.]
[This entry is marked for removal.]GE Medical Systems and Amersham announced in April 2004 the completion of a share exchange acquisition of Amersham Health by GE. The result of this acquisition is the new GE Healthcare, based in the UK, totally owned by General Electric (GE). The British company was a producer of contrast-imaging agents used to enhance image quality in X-ray, magnetic resonance imaging, and ultrasound procedures. It was also a leading producer of radiopharmaceuticals used in nuclear medicine imaging. Amersham Health was the firm's imaging, diagnostics, and therapeutics segment. Amersham was involved in biotechnology research through its Amersham Biosciences unit, which makes scanners, sequencers, microarrays, industrial separations, and other research supplies. Result Pages : |
Medical-Ultrasound-Imaging.com
former US-TIP.com
Member of SoftWays' Medical Imaging Group - MR-TIP • Radiology TIP • Medical-Ultrasound-Imaging
Copyright © 2008 - 2024 SoftWays. All rights reserved.
Terms of Use | Privacy Policy | Advertise With Us
former US-TIP.com
Member of SoftWays' Medical Imaging Group - MR-TIP • Radiology TIP • Medical-Ultrasound-Imaging
Copyright © 2008 - 2024 SoftWays. All rights reserved.
Terms of Use | Privacy Policy | Advertise With Us
[last update: 2023-11-06 01:42:00]




